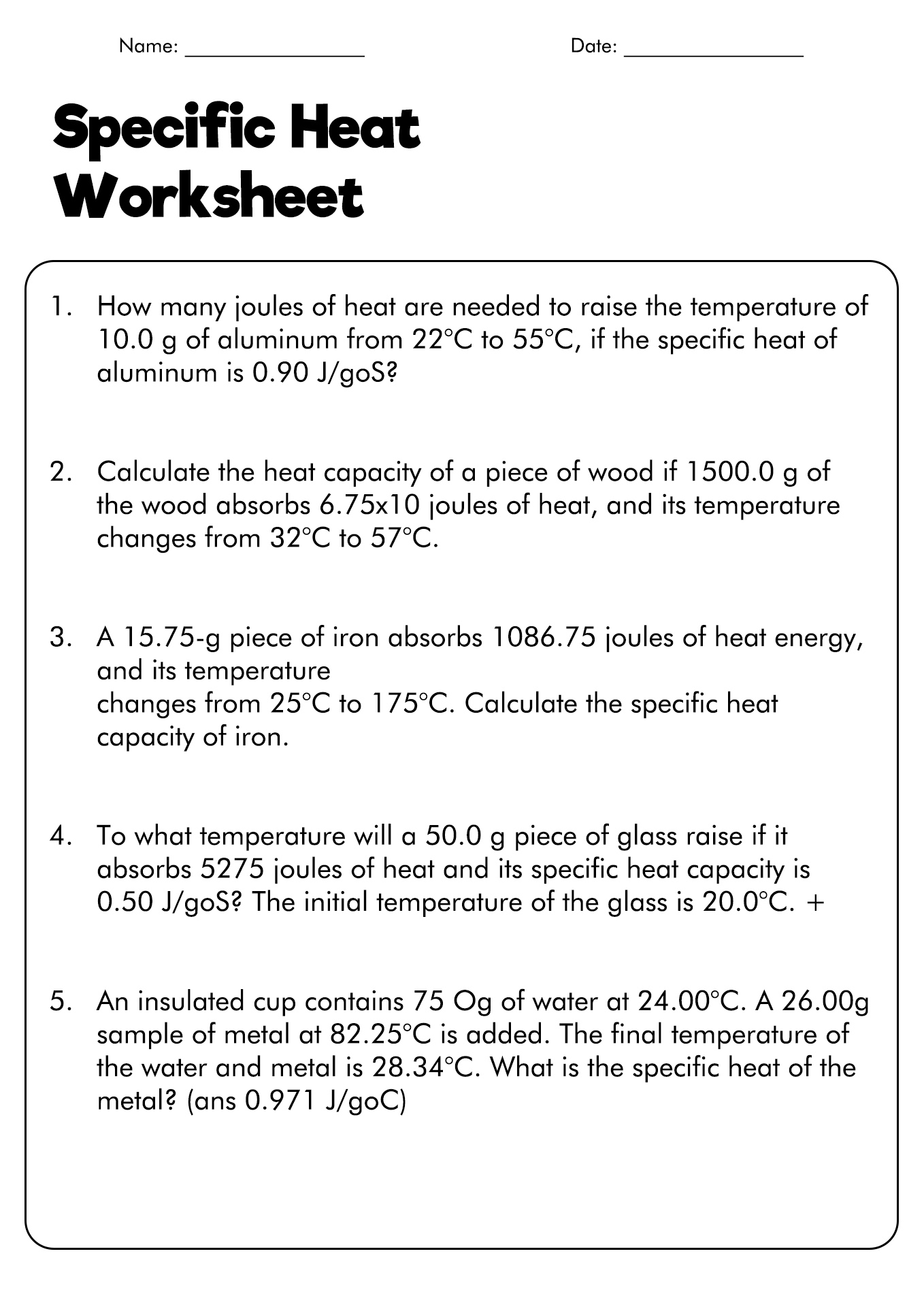
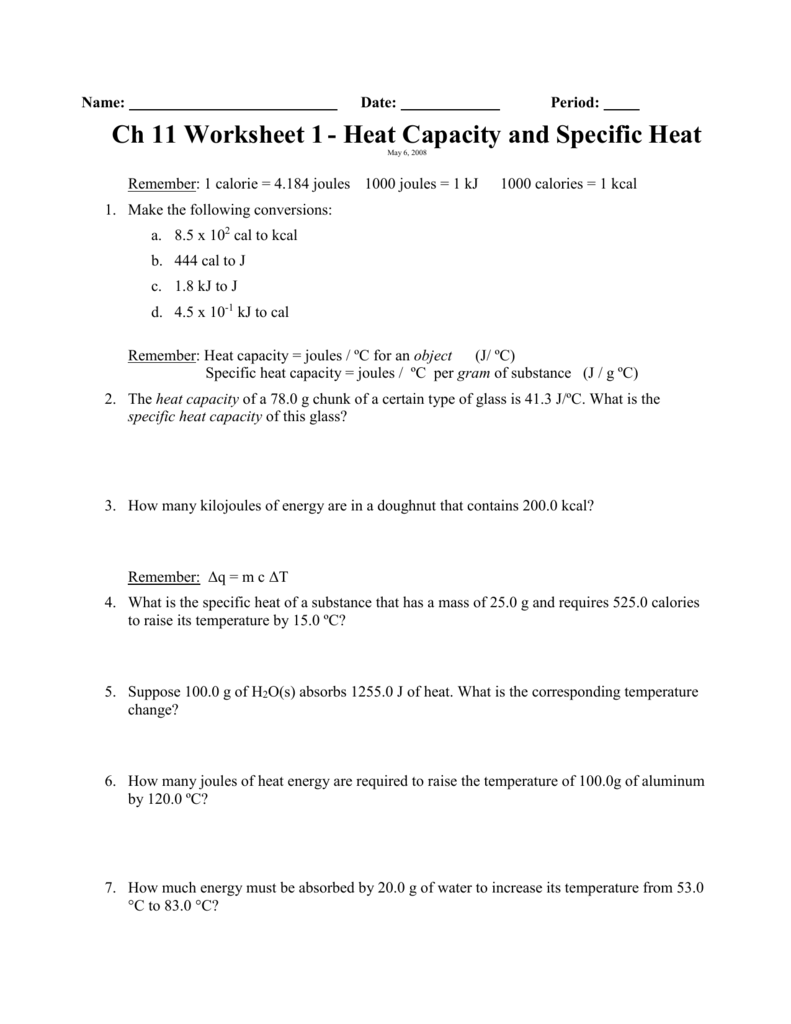
Specific Heat And Heat Capacity Worksheet Answers - It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil? Calculate the specific heat capacity of iron. How much energy was used to heat cu? How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. 5.0 g of copper was heated from 20°c to 80°c. Heat practice problems q = m x ∆t x c 1. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car.
How much energy was used to heat cu? Calculate the specific heat capacity of iron. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. 5.0 g of copper was heated from 20°c to 80°c. What does it mean if water has a higher specific heat capacity than oil? It requires more energy to raise its temperature by the same amount. Heat practice problems q = m x ∆t x c 1. Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to.
Calculate the specific heat capacity of iron. How much energy was used to heat cu? Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. 5.0 g of copper was heated from 20°c to 80°c. What does it mean if water has a higher specific heat capacity than oil? It requires more energy to raise its temperature by the same amount. Heat practice problems q = m x ∆t x c 1.
Heat Capacity And Specific Heat Worksheet Heat Worksheet Wor
How much energy was used to heat cu? What does it mean if water has a higher specific heat capacity than oil? Heat practice problems q = m x ∆t x c 1. Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. How many joules of heat are needed.
Specific Heat Worksheets WorksheetsGO
Calculate the specific heat capacity of iron. What does it mean if water has a higher specific heat capacity than oil? Heat practice problems q = m x ∆t x c 1. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. It requires more energy to raise its temperature by.
Specific Heat Worksheet Answers 1
5.0 g of copper was heated from 20°c to 80°c. Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. Heat practice problems q = m x ∆t x c 1. How much energy was used to heat cu? How many joules of heat are needed to raise the temperature.
11 Science Heat Energy Worksheets With Answer Free PDF at
5.0 g of copper was heated from 20°c to 80°c. What does it mean if water has a higher specific heat capacity than oil? How much energy was used to heat cu? It requires more energy to raise its temperature by the same amount. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is.
Specific Heat Capacity Worksheet (Key) Specific Heat Capacity L. tL
Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. Calculate the specific heat capacity of iron. It requires more energy to raise its temperature by the same amount. How much energy was used to heat cu? What does it mean if water has a higher specific heat capacity than.
Solved Specific Heat and Heat Capacity Worksheet DIRECTIONS
Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a substance is the amount of heat required to raise the. It requires more energy to raise its temperature by the same amount. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. 5.0 g of copper was.
Specific Heat Worksheet Answers
Calculate the specific heat capacity of iron. How much energy was used to heat cu? Heat practice problems q = m x ∆t x c 1. Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of a.
33 Specific Heat Capacity Worksheet Answers support worksheet
How much energy was used to heat cu? It requires more energy to raise its temperature by the same amount. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Heat practice problems q = m x ∆t x c 1. Answers to worksheet # 17 calculating heat the specific heat.
Specific Heat Capacity Worksheet
What does it mean if water has a higher specific heat capacity than oil? 5.0 g of copper was heated from 20°c to 80°c. Heat practice problems q = m x ∆t x c 1. It requires more energy to raise its temperature by the same amount. Answers to worksheet # 17 calculating heat the specific heat capacity (c) of.
Heat Capacity and Specific Heat Calculations Practice A 1 g
It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil? Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. How many joules of heat are needed to raise the temperature of 10.0 g.
What Does It Mean If Water Has A Higher Specific Heat Capacity Than Oil?
How much energy was used to heat cu? Engineers use specific heat capacity when determining which materials to use in the construction of buildings, rockets, and even car. 5.0 g of copper was heated from 20°c to 80°c. It requires more energy to raise its temperature by the same amount.
Answers To Worksheet # 17 Calculating Heat The Specific Heat Capacity (C) Of A Substance Is The Amount Of Heat Required To Raise The.
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Heat practice problems q = m x ∆t x c 1.