Molnupiravir Patient Fact Sheet - Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Pediatric (for additional information see molnupiravir (united states: Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web signs of an allergic reaction, like rash; Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. Red, swollen, blistered, or peeling skin with or without fever; Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: To account for the fda.
Web signs of an allergic reaction, like rash; Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Pediatric (for additional information see molnupiravir (united states: To account for the fda. Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Red, swollen, blistered, or peeling skin with or without fever; Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons:
Web signs of an allergic reaction, like rash; Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Pediatric (for additional information see molnupiravir (united states: Red, swollen, blistered, or peeling skin with or without fever; Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. To account for the fda.
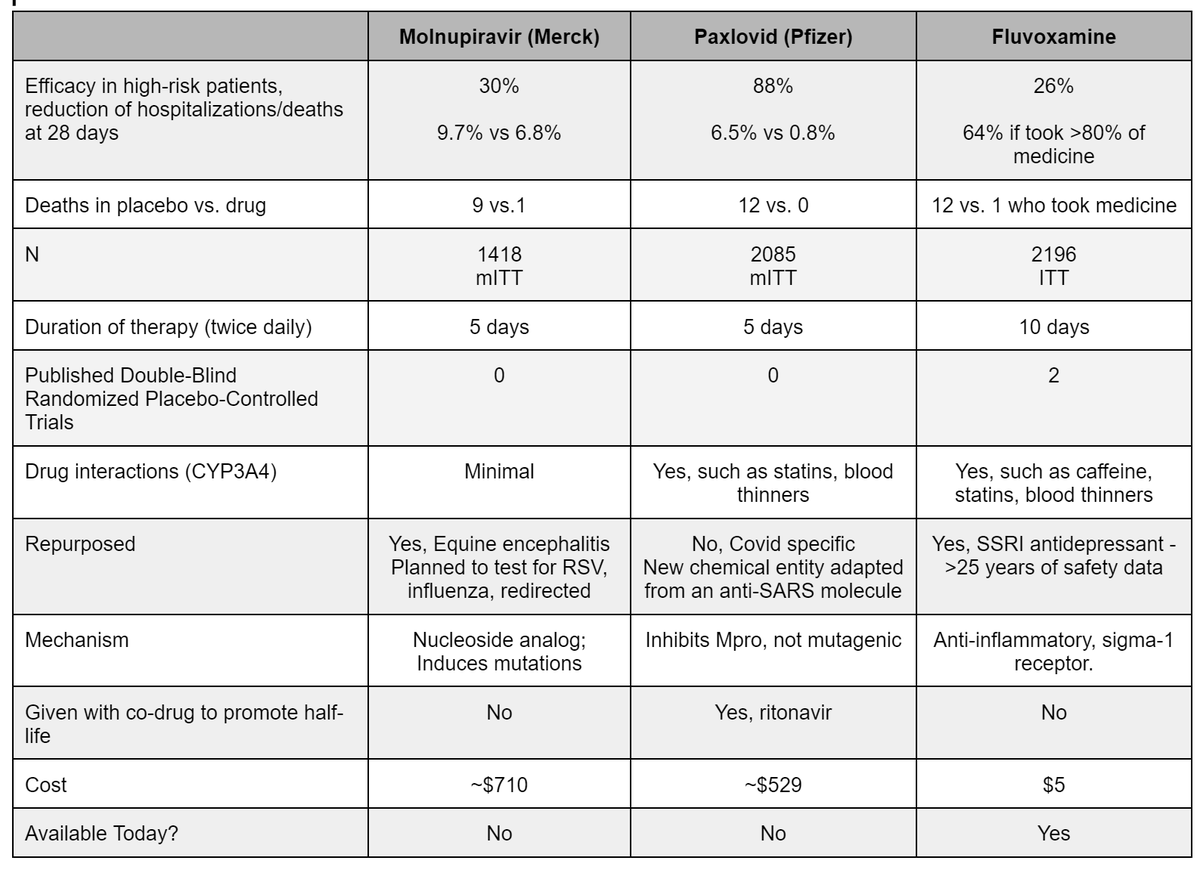
Updated chart on early COVID19 treatment for Molnupiravir,
Red, swollen, blistered, or peeling skin with or without fever; To account for the fda. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Food and drug administration (fda) has issued an eua for the emergency.
Molnupiravir Fact Sheet for Healthcare Givers and Recipients
Pediatric (for additional information see molnupiravir (united states: Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. To account for the fda. Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following.
Patient Handouts — TeamTAUC
Pediatric (for additional information see molnupiravir (united states: Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Web signs of an allergic reaction, like rash; Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Food and drug administration (fda) has issued an eua for the.
Test and Treat COVID19 Community Guide to Molnupiravir Treatment
To account for the fda. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Red, swollen, blistered, or peeling skin with or without fever; Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web signs of an allergic reaction, like rash;
Molnupiravir STELLA 400 mg GONSA
Red, swollen, blistered, or peeling skin with or without fever; Web signs of an allergic reaction, like rash; Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Pediatric (for additional information see molnupiravir (united states:
Merck’s Molnupiravir Second COVID Pill Is SecondLine, But Timing
Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. Web signs of an allergic reaction, like rash; Pediatric (for additional information see molnupiravir (united states: Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web fact sheet for patients and caregivers emergency use authorization (eua) of.
Molnupiravir (new antiviral) Interim Data Unambiguous Science
Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web signs of an allergic reaction, like rash; To account for the fda. Red, swollen, blistered, or peeling skin with or without fever; Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for.
A Comparison Of Paxlovid Versus Molnupiravir The First Oral COVID
Web signs of an allergic reaction, like rash; Pediatric (for additional information see molnupiravir (united states: Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Web fact sheet for patients and caregivers emergency use authorization (eua).
Today MOLNUPIRAVIR Medication News, Jan 28, 2022
Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. To account for the fda. Pediatric (for additional information see molnupiravir (united states: Red, swollen, blistered, or peeling skin with or without fever;
Medicines factsheets Healthify
Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web the molnupiravir (mov) eua factsheets are being revised at this time for the following two reasons: Food and drug administration (fda) has issued an eua for the.
Red, Swollen, Blistered, Or Peeling Skin With Or Without Fever;
Food and drug administration (fda) has issued an eua for the emergency use of the unapproved. To account for the fda. Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for. Web signs of an allergic reaction, like rash;
Web The Molnupiravir (Mov) Eua Factsheets Are Being Revised At This Time For The Following Two Reasons:
Pediatric (for additional information see molnupiravir (united states: Web fact sheet for patients and caregivers emergency use authorization (eua) of lagevriotm (molnupiravir) capsules for.