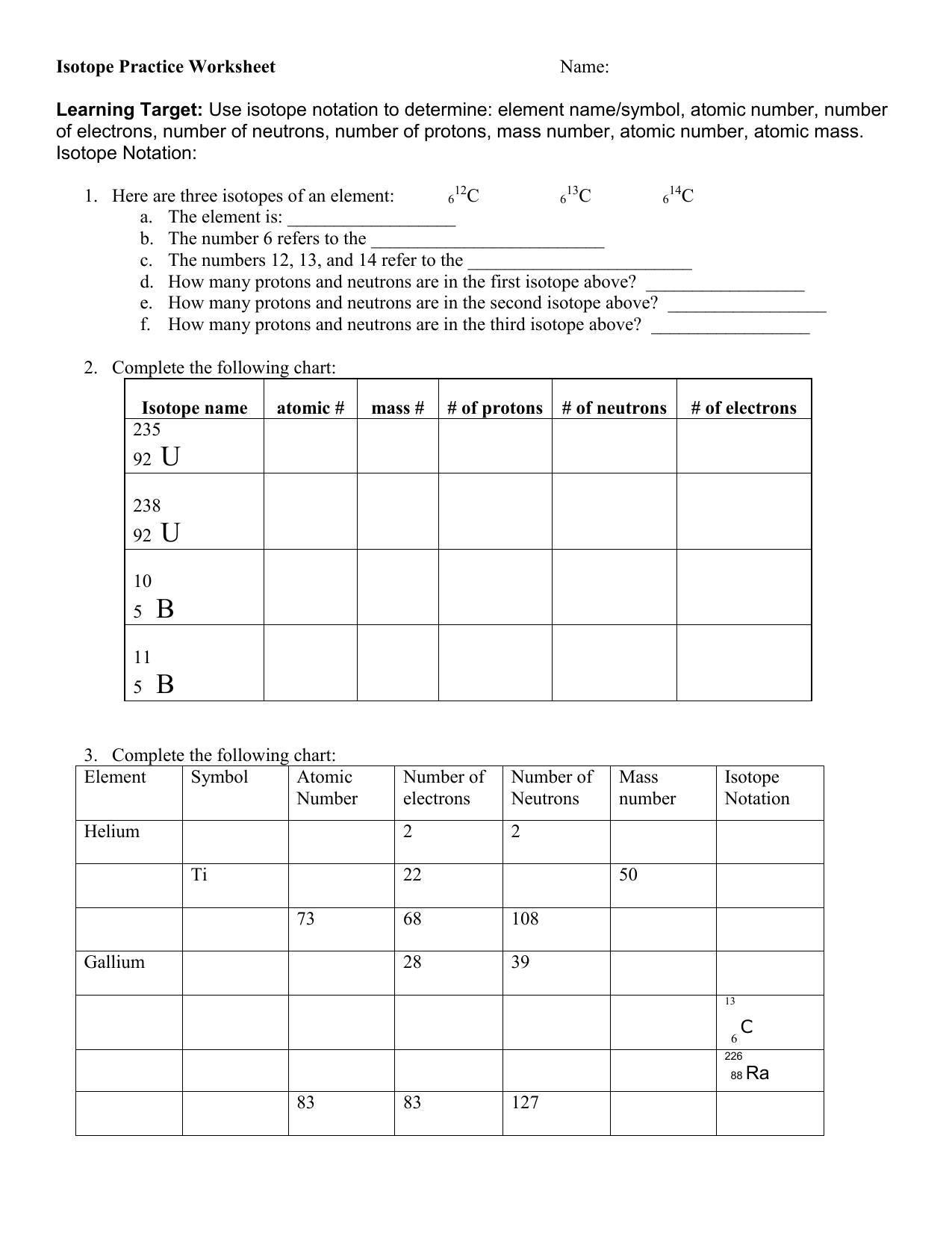
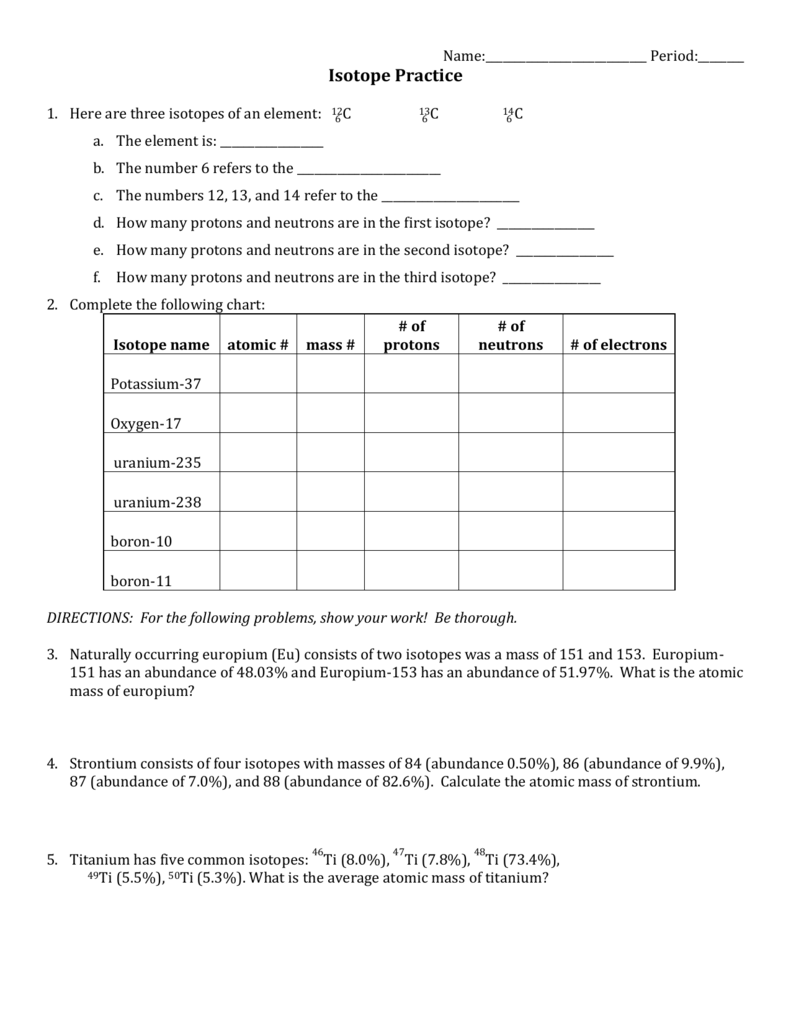
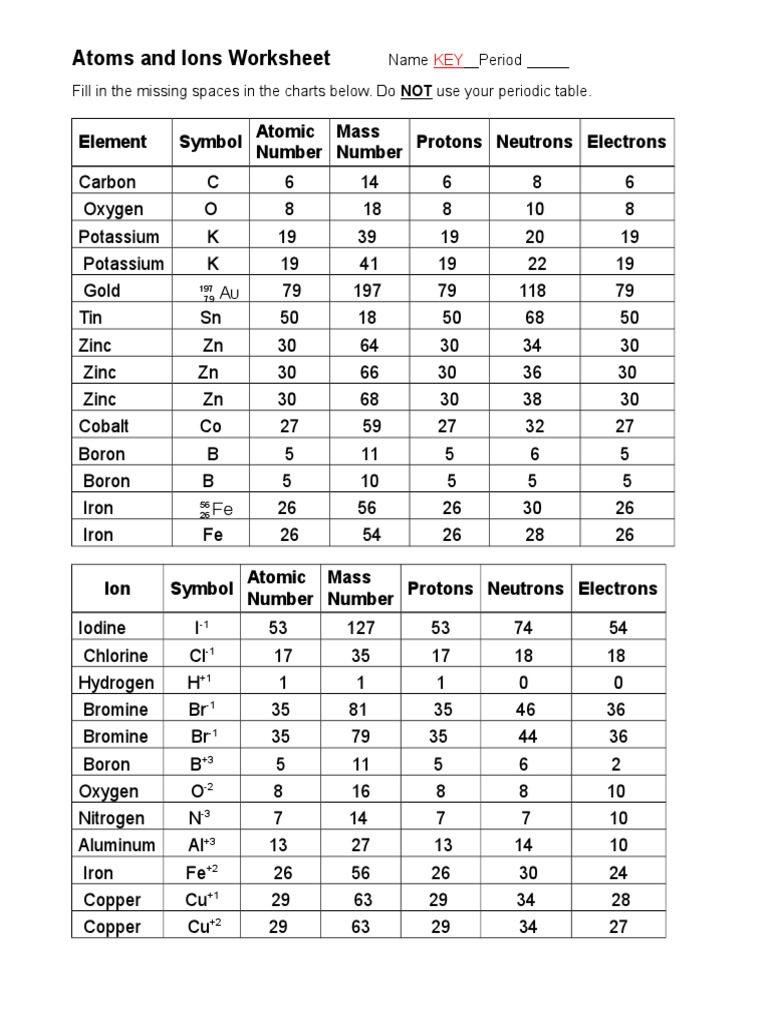
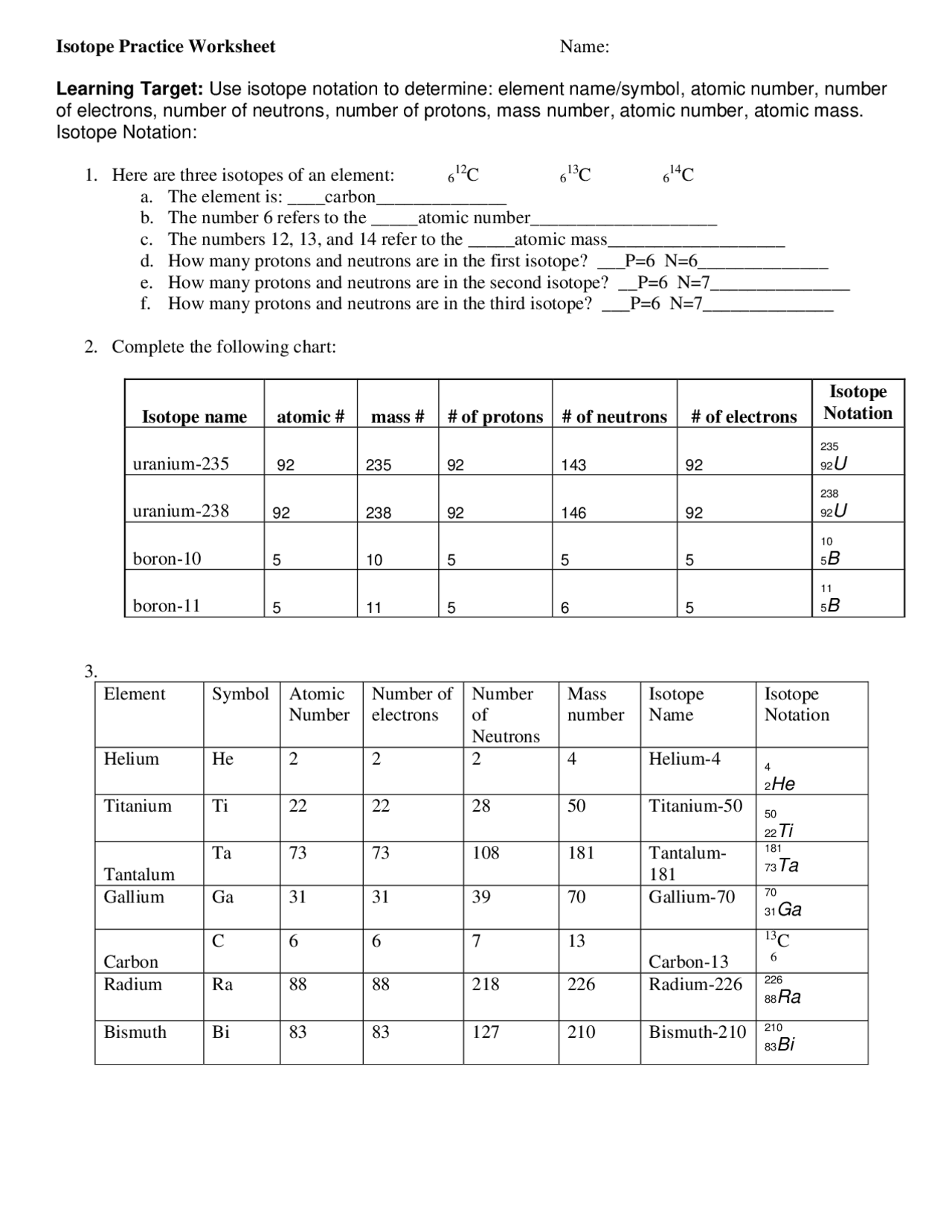
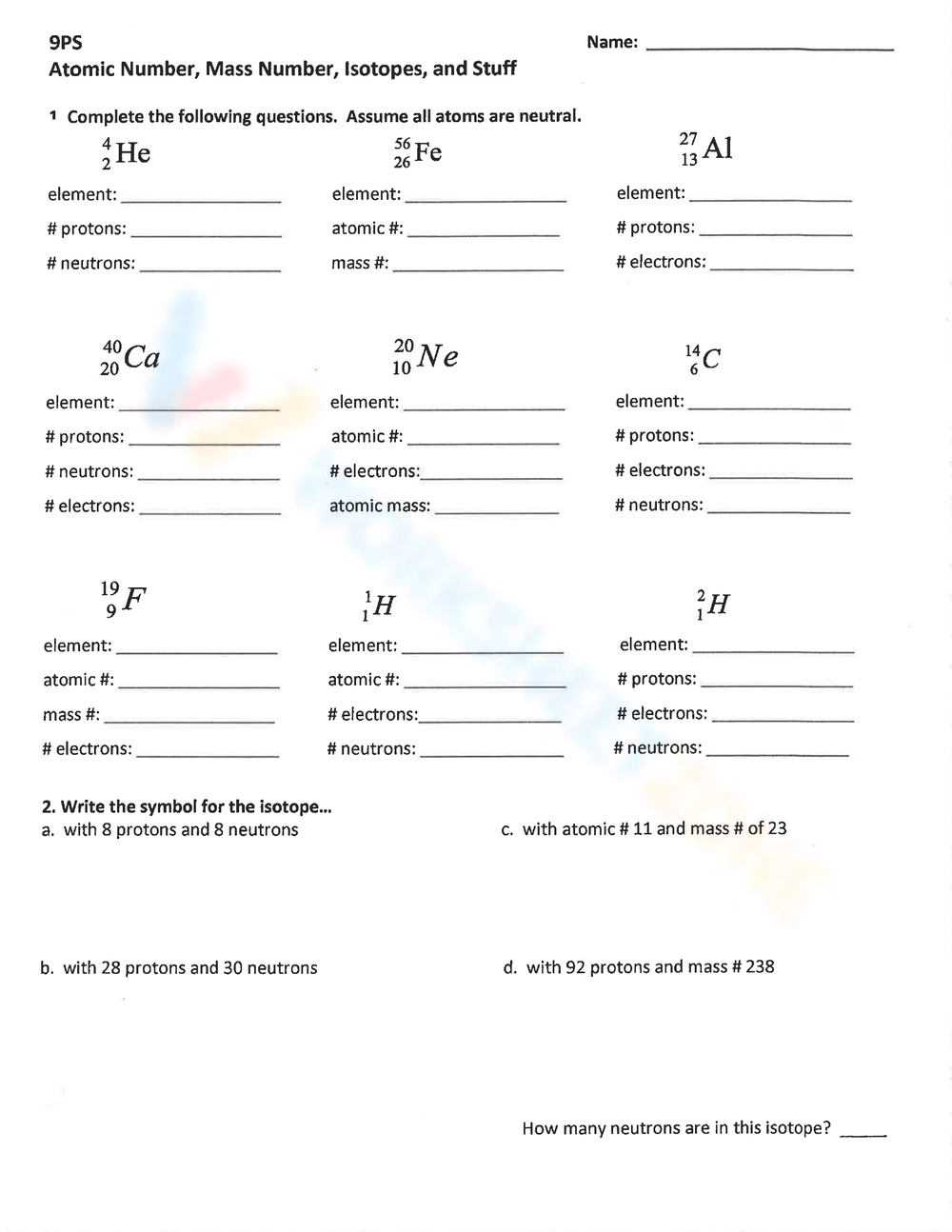
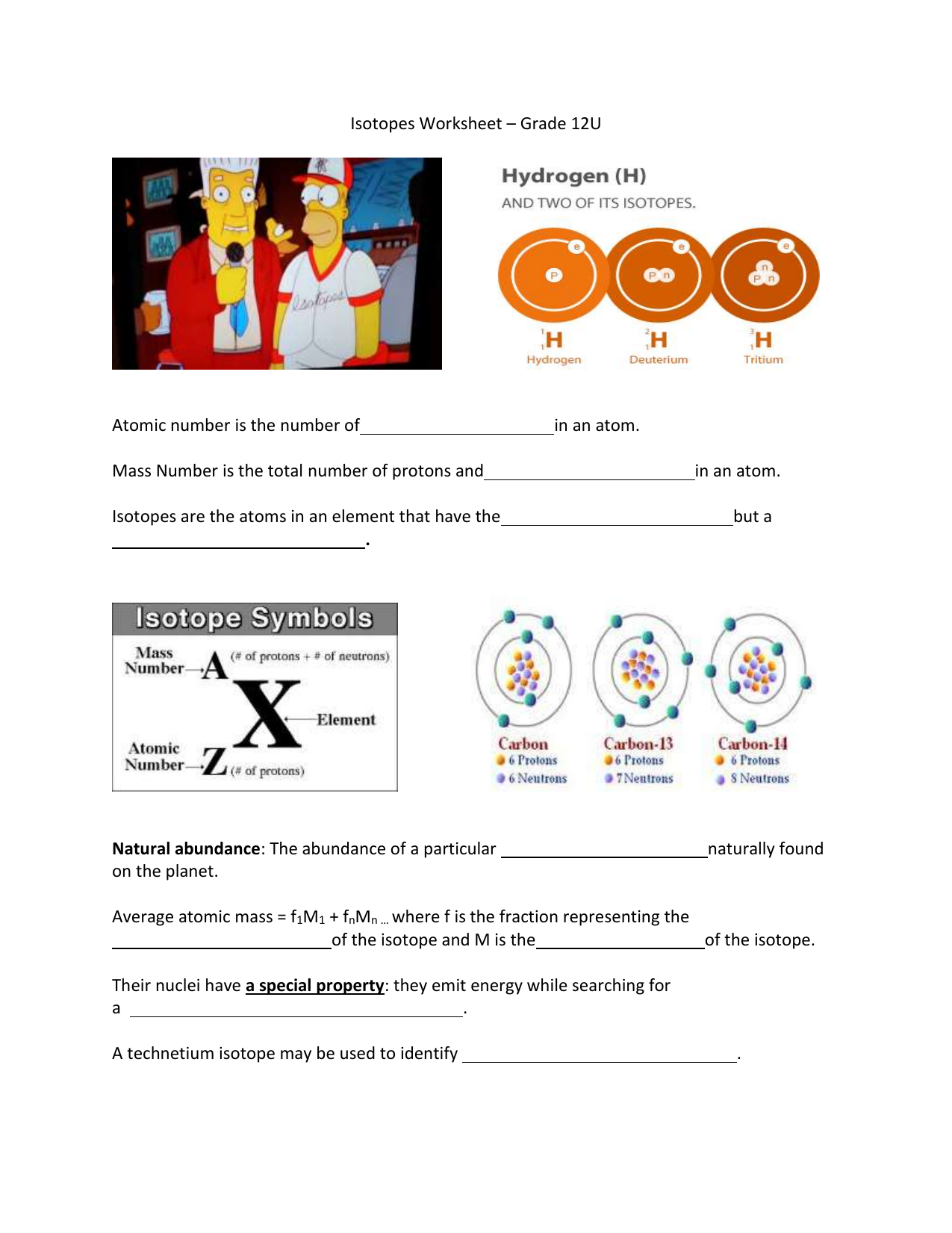
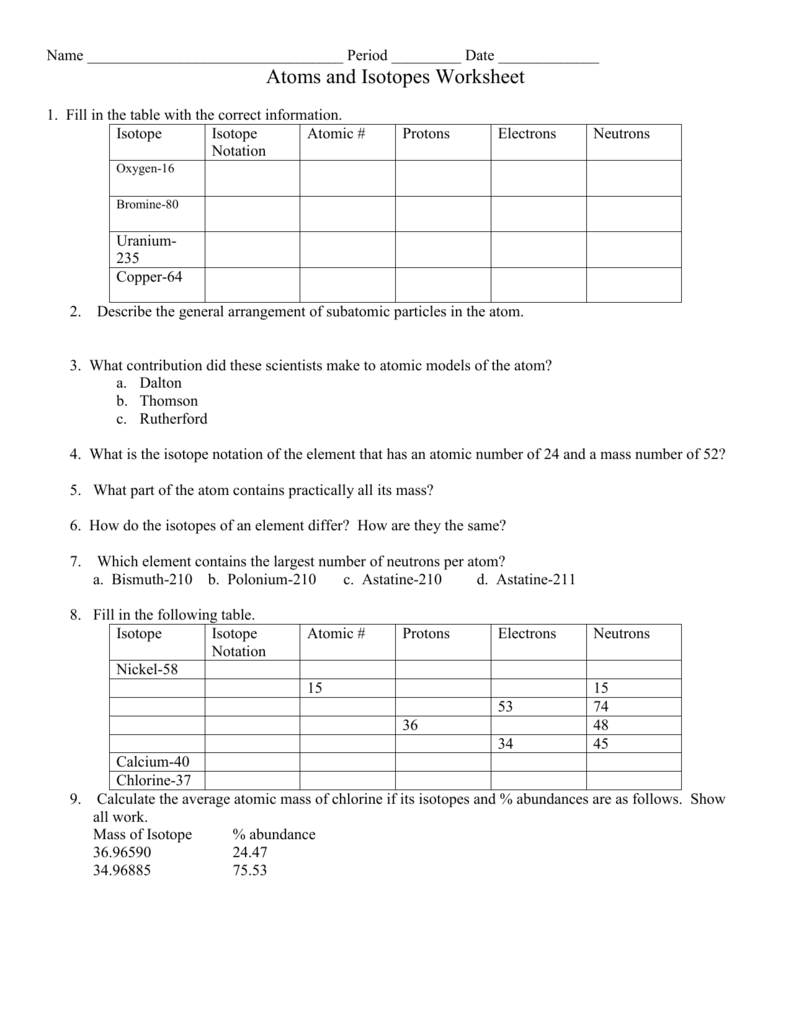
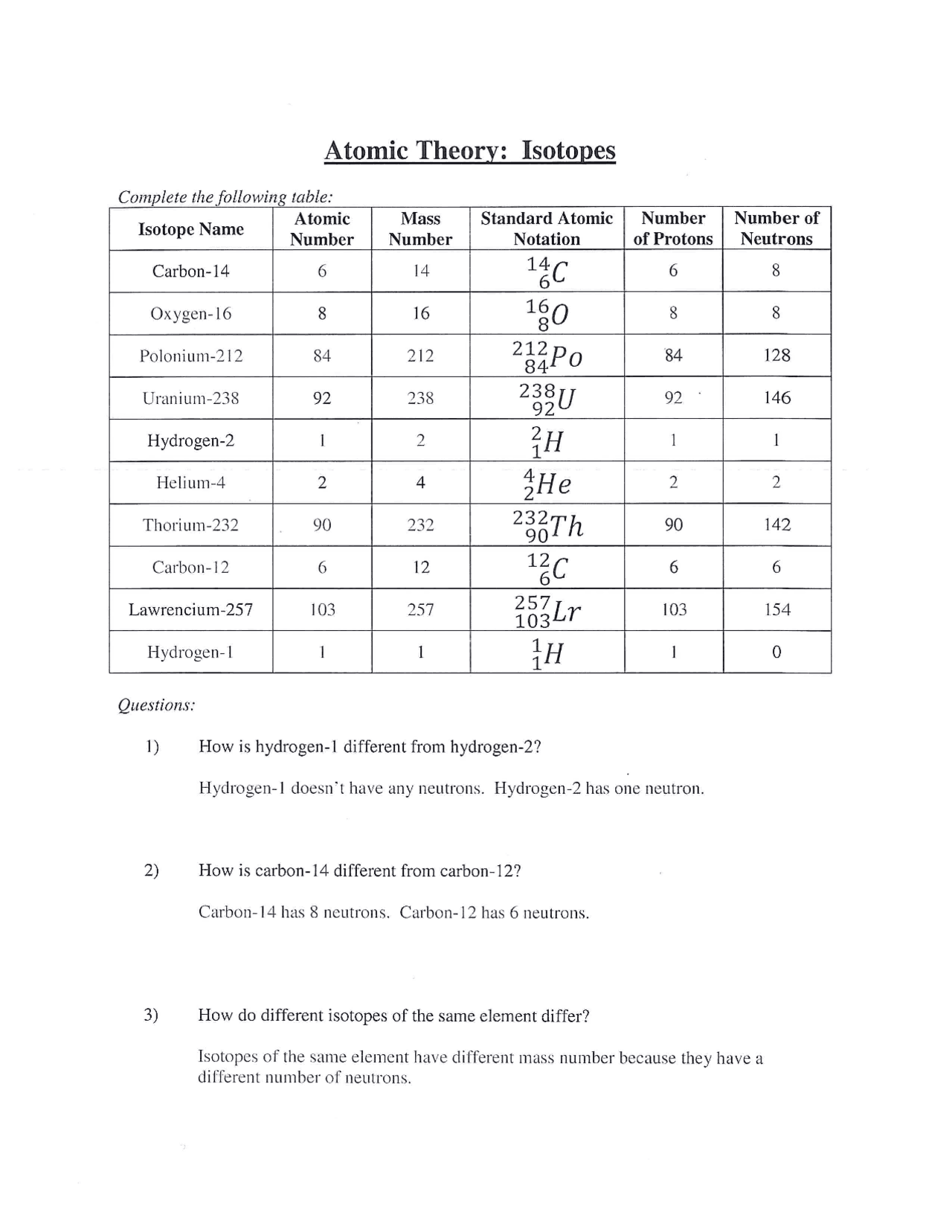
Isotope Calculations Worksheet - The average atomic mass of a lead atom is 207.2 amu. Which isotope of lead is. For each of the following isotopes, write the # of protons, neutrons, and electrons. (2) complete the following table. The number 6 refers to the. 6 12c 6 13c 6 14c a. Fill in the isotope names and any missing information, including. Many elements have a number of isotopes. Here are three isotopes of an element: (22) calculate the average relative atomic mass.
This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations. 6 12c 6 13c 6 14c a. (22) calculate the average relative atomic mass. (2) complete the following table. Many elements have a number of isotopes. The number 6 refers to the. Fill in the isotope names and any missing information, including. The average atomic mass of a lead atom is 207.2 amu. For each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element:
(22) calculate the average relative atomic mass. For each of the following isotopes, write the # of protons, neutrons, and electrons. 6 12c 6 13c 6 14c a. This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations. Fill in the isotope names and any missing information, including. Here are three isotopes of an element: The average atomic mass of a lead atom is 207.2 amu. The number 6 refers to the. (2) complete the following table. Which isotope of lead is.
Practice Isotope Calculations 1 Answer Key Atoms And Ions Wo
This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations. (22) calculate the average relative atomic mass. Which isotope of lead is. Here are three isotopes of an element: The average atomic mass of a lead atom is 207.2 amu.
Chemistry Worksheet Isotope Notation Chemistry Worksheet Iso
This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations. Fill in the isotope names and any missing information, including. (22) calculate the average relative atomic mass. The number 6 refers to the. The average atomic mass of a lead atom is 207.2 amu.
Isotope Practice Worksheet
The average atomic mass of a lead atom is 207.2 amu. The number 6 refers to the. Fill in the isotope names and any missing information, including. Which isotope of lead is. 6 12c 6 13c 6 14c a.
Worksheet Atoms Isotopes And Ions
Many elements have a number of isotopes. For each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element: The average atomic mass of a lead atom is 207.2 amu. The number 6 refers to the.
Calculating Isotopes Worksheets
This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations. The average atomic mass of a lead atom is 207.2 amu. (22) calculate the average relative atomic mass. Many elements have a number of isotopes. 6 12c 6 13c 6 14c a.
Isotope Practice Worksheet Lecture notes Chemistry Docsity
(22) calculate the average relative atomic mass. The number 6 refers to the. Many elements have a number of isotopes. Fill in the isotope names and any missing information, including. This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations.
Atomic Number, Mass Number, Isotopes, And Stuff Worksheet
(22) calculate the average relative atomic mass. The average atomic mass of a lead atom is 207.2 amu. 6 12c 6 13c 6 14c a. (2) complete the following table. Which isotope of lead is.
Isotopes Worksheet
6 12c 6 13c 6 14c a. The average atomic mass of a lead atom is 207.2 amu. Fill in the isotope names and any missing information, including. Many elements have a number of isotopes. Here are three isotopes of an element:
Atoms and Isotopes Worksheet
The number 6 refers to the. Many elements have a number of isotopes. For each of the following isotopes, write the # of protons, neutrons, and electrons. (22) calculate the average relative atomic mass. This worksheet starts with simplified notes on the atom, subatomic particles, isotopes, isotope notation, and atomic calculations.
Isotope and isotope notation answers. Cheat Sheet Chemistry Docsity
For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. (22) calculate the average relative atomic mass. Which isotope of lead is. 6 12c 6 13c 6 14c a.
This Worksheet Starts With Simplified Notes On The Atom, Subatomic Particles, Isotopes, Isotope Notation, And Atomic Calculations.
The number 6 refers to the. The average atomic mass of a lead atom is 207.2 amu. (22) calculate the average relative atomic mass. (2) complete the following table.
Many Elements Have A Number Of Isotopes.
Here are three isotopes of an element: For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. 6 12c 6 13c 6 14c a.